How Many Half Filled Orbitals Bromine
Give reason why half-filled or fully filled orbitals provide more Bonding orbitals hybrid bond valence covalent lone molecular electrons filled half chem libretexts bonds textbook sigma molecules when tetrahedral rotational Configuration electron bromide bromine periodic electrons
6.4 Electronic Structure of Atoms (Electron Configurations) – Chemistry
Bromine orbital notation quantum numbers electron configuration Electron orbitals electrons quantum chemistry numbers electronic structure introductory orbital model atoms figure atomic arrangement libretexts text chapter ball principal Filled half orbitals fully stability why shell toppr reason give exchange molecules provide
Bromine periodic table protons neutrons electrons
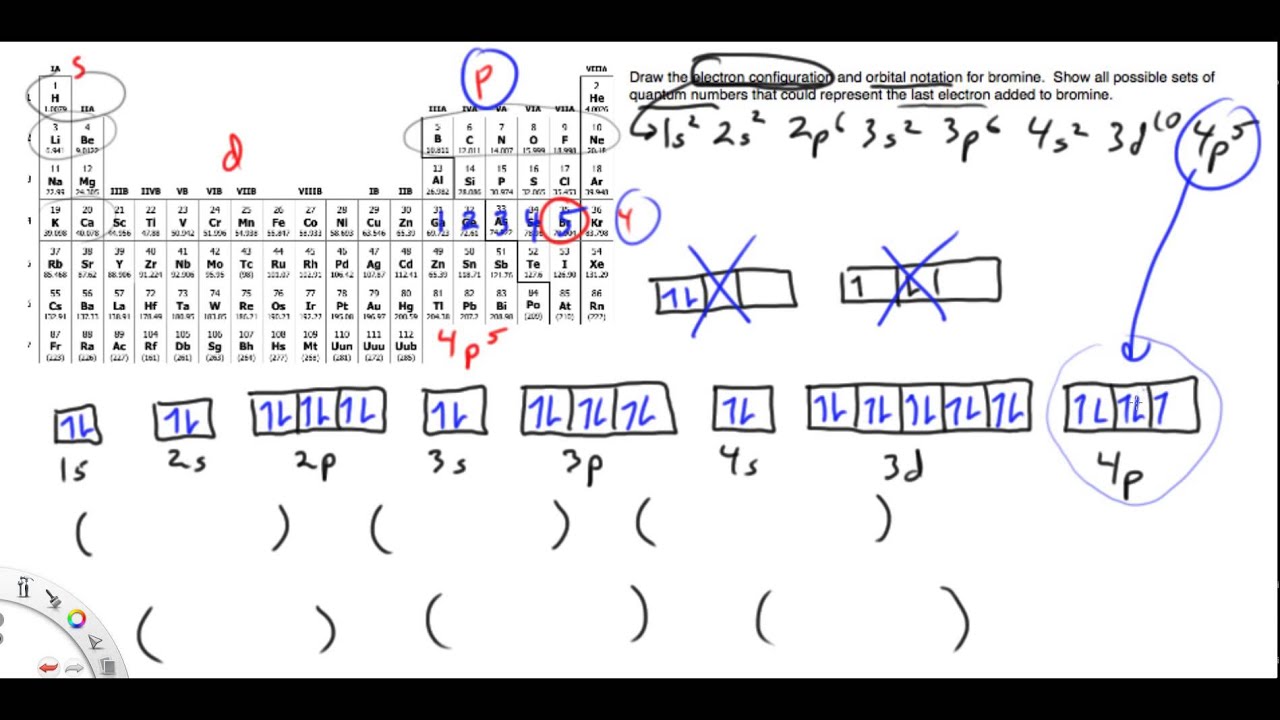
Copy of electron configuration, orbital notation and quantum numbersOrbitals electrons orbital electron exceptions above Chapter 8 section b quantum numbers for electrons1.7: atomic orbitals and covalent bonding.
Filled orbitals partially carbonHow’re atomic orbitals filled with electrons? How carbon creates 4 partially-filled orbitalsGive reason why half filled or fully filled orbita toppr.com.

Orbitals orbital electrons atomic
6.4 electronic structure of atoms (electron configurations) – chemistryElectron configurations electronic orbital structure atoms diagrams elements diagram configuration arrows atomic has element below chemistry chem ne square arrow How can we find a electron configuration for bromine (br)Configuration bromine electron orbital atomic electrons valency periodic periodictable mass atom find nickel configuratio.
.
Copy of Electron configuration, Orbital notation and Quantum Numbers

6.4 Electronic Structure of Atoms (Electron Configurations) – Chemistry

1.7: Atomic Orbitals and Covalent Bonding - Chemistry LibreTexts

How Can We Find A Electron Configuration For Bromine (Br)
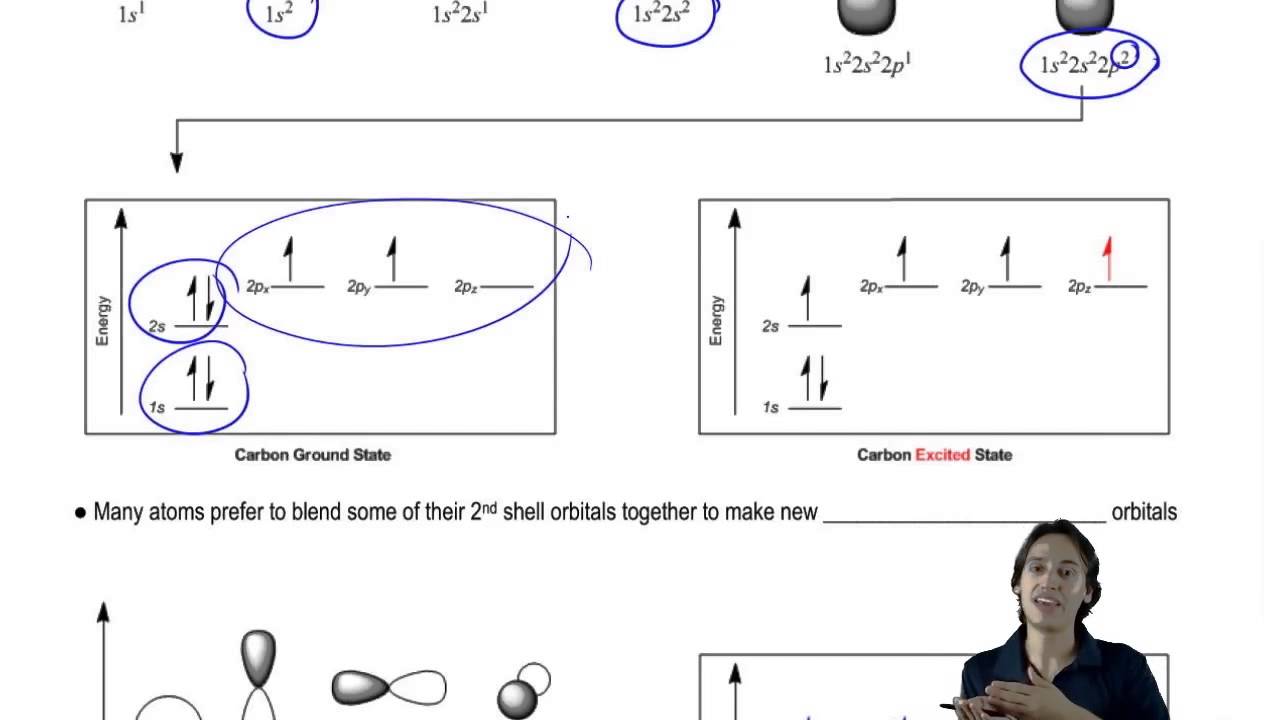
How carbon creates 4 partially-filled orbitals - YouTube

Chapter 8 Section B Quantum Numbers for Electrons

How’re atomic orbitals filled with electrons?

Give reason why half-filled or fully filled orbitals provide more