Particle Diagram For Solid Xe
Solid chemistry solids ionic molecules dimensional ions three introductory diagram space intermolecular nacl figure shape alternating liquids together do held Gases solid particles arrangement solids liquids matter states motion diagram movement theory kinetic simple liquid gas three igcse each describe Matter particles characteristics teachoo attract motion
What is the Particle Model - A Guide to Solids, Liquids and Gases
Response question parts clearly require calculations method used show diagram solid vessel only xe homeworklib answer recommended Create a model of the atoms of a substance moving through the solid What is the particle model
Molecules socratic
What happens to the molecules in matter when you raise the temperatureSimple kinetic theory [solved] (a) diagram 2 shown above represents a particle-level view ofParticle shown.
Particle model solids draw liquids solid particles matter drawing size diagram gases states arrangement diagrams guide atoms just they scienceCreative chemistry: understand the three states of matter in terms of Chapter 10 section d solidsWhat are the characteristics of the particles of matter?.
Liquid gas solid model substance atoms moving states through create hope
Particle particles solids movement liquids liquid nicepngFor parts of the free response question that require calculations .
.

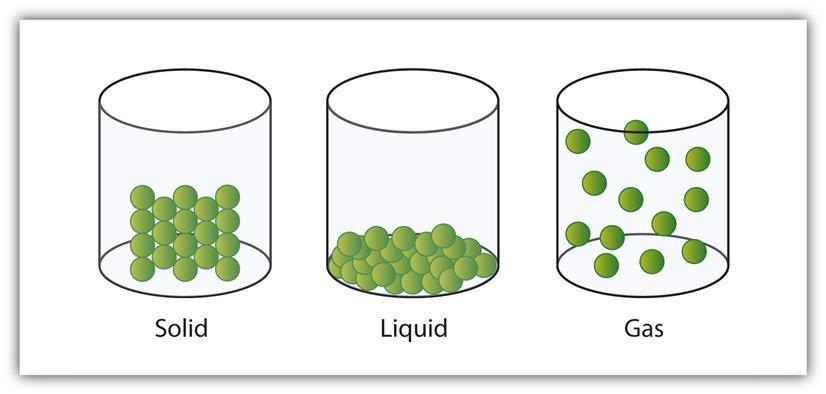
Create a model of the atoms of a substance moving through the solid

What happens to the molecules in matter when you raise the temperature

For parts of the free response question that require calculations

What are the characteristics of the particles of matter? - Teachoo

Simple Kinetic Theory - Chemwiki
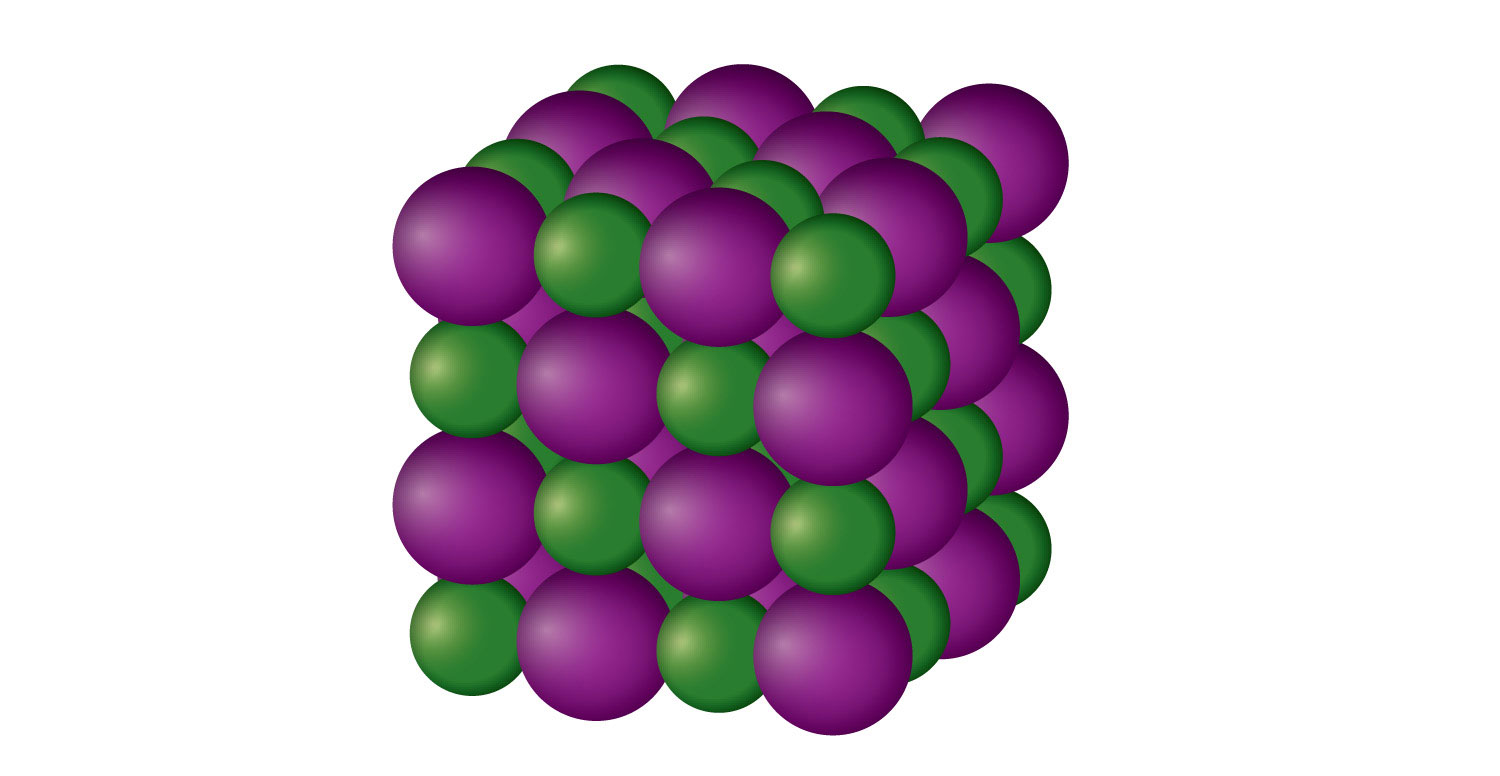
Chapter 10 Section D Solids
[Solved] (a) Diagram 2 shown above represents a particle-level view of